 Covid-19
Covid-19
Hetero launches Favipiravir in India to treat mild to moderate Covid-19
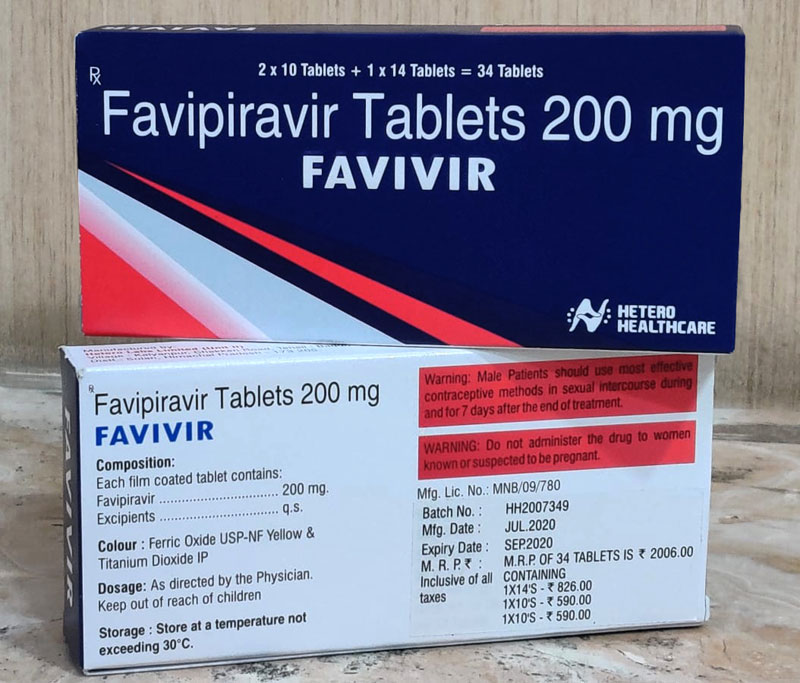
Kolkata/IBNS: Hetero, one of India’s leading generic pharmaceutical companies and the world’s largest producer of antiretroviral drugs, has announced the launch of generic Favipiravir in India under the brand name ‘Favivir' to treat mild to moderate Covid-19.
Hetero has been granted the manufacturing and marketing approval for Favipiravir from the Drug Controller General of India (DCGI).
Favivir is the second drug developed by Hetero after Covifor (Remdesivir) used in the treatment of Covid-19.
It is an oral antiviral medication that has demonstrated positive clinical outcomes.
Favivir improves treatment accessibility to a significant amount of Covid-19 patient population, which usually sustains mild to moderate symptoms.
Hetero’s Favivir is priced at Rs. 59 per tablet and is marketed and distributed by Hetero Healthcare Limited.
The product is available at all retail medical outlets and hospital pharmacies across the country and will be sold only on prescription.
Backed by strong vertical integration capabilities, the drug is being manufactured at ourworld-class formulation facility in India, which has been approved by stringent global regulatory authorities such as USFDA and the EU, among others.
Support Our Journalism
We cannot do without you.. your contribution supports unbiased journalism
IBNS is not driven by any ism- not wokeism, not racism, not skewed secularism, not hyper right-wing or left liberal ideals, nor by any hardline religious beliefs or hyper nationalism. We want to serve you good old objective news, as they are. We do not judge or preach. We let people decide for themselves. We only try to present factual and well-sourced news.







