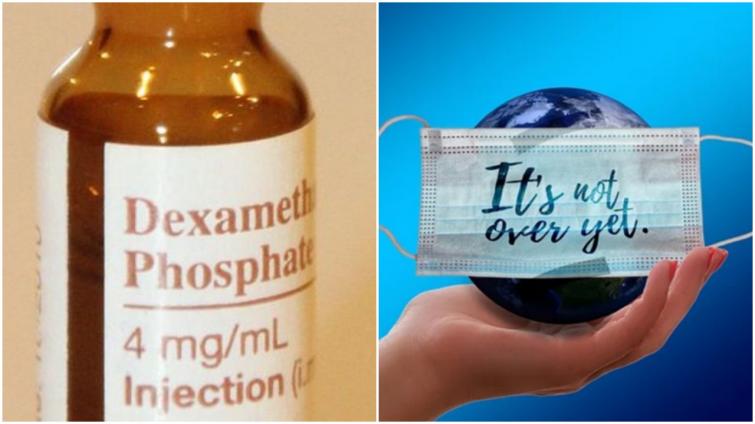
India allows use of dexamethasone for COVID-19 treatmentÂ
New Delhi/IBNS: The Indian government on Saturday allowed the use of steroid drug dexamethasone for COVID-19 patients.
"Keeping pace with evolving knowledge about COVID-19, especially in terms of effective drugs, the Union Ministry of Health & Family Welfare has today released an updated clinical management protocol for managing COVID-19 cases," read a statement issued by the Indian government.
The updated protocol includes the advice to use Dexamethasone as an alternative choice to Methylprednisolone for managing moderate to severe cases of COVID-19.
The change has been made after considering the latest available evidence and expert consultation.
Dexamethasone is a corticosteroid drug used in a wide range of conditions for its anti-inflammatory and immunosuppressant effects.
"The drug has been tested in hospitalized patients with COVID-19 in the RECOVERY clinical trial and was found to have benefits for critically ill patients and has been shown to reduce mortality by about one third for patients on ventilators, and by about one fifth for patients being maintained on oxygen therapy. The drug is also a part of the National List of Essential Medicines (NLEM) and is widely available," read the statement.
Support Our Journalism
We cannot do without you.. your contribution supports unbiased journalism
IBNS is not driven by any ism- not wokeism, not racism, not skewed secularism, not hyper right-wing or left liberal ideals, nor by any hardline religious beliefs or hyper nationalism. We want to serve you good old objective news, as they are. We do not judge or preach. We let people decide for themselves. We only try to present factual and well-sourced news.







